Platelets are normally transfused either prophylactically for oncology and hematology patients, or to help stop severe bleeding. Since the mid-1980s, platelets have been stored at room-temperature with constant agitation. However, recent and past research suggests that the cold storage of platelets actually preserves their hemostatic function, which may be best for actively bleeding patients. The FDA defines cold-stored platelets as those stored continuously at 1 to 6 degrees Celsius within a specified time after collection. It is stored for up to 14 days from the date of collection when such apheresis platelets are intended for the treatment of active bleeding when conventional platelets are not available or their use is not practical.
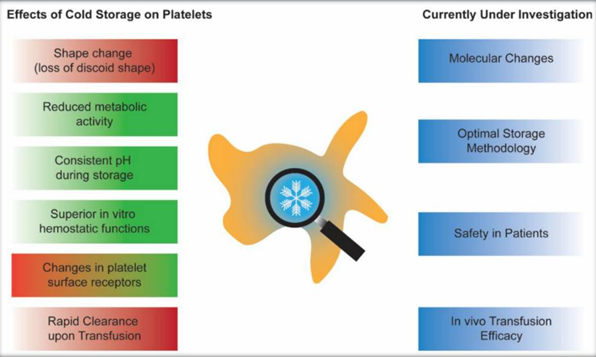
Cold-stored platelets (CPs) are not a new product. Prior to the late 1970s, all platelet units used for transfusions were stored at 4 °C. However, it was recognized in 1969 that CPs were quickly cleared from circulations, whereas room-temperature stored platelets (RPs) had significantly longer circulation times [1] The rapid clearance of CPs is attributed to one of the significant physiological changes that occur when platelets are exposed to old temperature. It was discovered that cold storage induces the clustering of platelet surface glycoprotein GPIb?, which has been shown to mediate phagocytosis by macrophages in short-term (<48 h) CPs [2,]. Long-term cold-stored platelets (>48 h) are cleared by hepatocytes in the liver through interaction of the Ashwell–Morell receptor with the exposed ?-GlcNAc moieties on CPs
Efforts to prevent the rapid clearance of CPs by galactosylation of the ?-GlcNAc moieties have not been successful in humans. Since the majority of platelet transfusions were given to patients with hematological disorders or thrombocytopenic patients in the late 1970s, there was a complete shift in platelet storage methodology from CPs to RPs. Cold temperatures also induce activation of the platelets in the form of platelet granule content release and the exposure of phosphatidylserine. One possible mechanism of cold-induced platelet activation is the significant accumulation of intracellular calcium during cold storage.Furthermore, cold temperatures have significant effects on the metabolism of stored platelets. CPs have reduced glucose consumption and lactate production. Storage in the cold has also been associated with the preservation of the mitochondrial functions of the platelets.
Despite the rapid in vivo clearance, there has been a renewed interest in bringing back CPs as a product. This recent resurgence can be attributed to three main reasons. First, cold storage significantly reduces the growth of microorganisms, such as bacteria, in the platelet units. This allows the platelet units to have a longer shelf life, thus reducing the wastage due to outdating. Second, CPs are shown to be hemostatically superior to RPs. Lastly, while RPs require constant mechanical agitation during storage, CPs do not seem to require agitation. This eliminates the cost of mechanical shakers and improves logistics handling during platelet shipping. Furthermore, recent investigations into the utilization of platelets for transfusion have shown that there is a significant increase in the need for therapeutic platelet transfusions compared to prophylactic transfusions. With superior hemostatic functions and a longer potential shelf life, CPs could be a promising product for actively bleeding patients.
During the late 1960s, when the platelet storage practice was switched from CPs to RPs, it was known that CPs were better for bleeding patients, as they showed superior correction in bleeding time. The latest in vitro models have revealed new information about the storage characteristics of CPs. Scanning electron microscopy was used to examine the characteristics of clots formed by CPs versus RPs. The microscopy images suggested that CPs had significantly more crosslinking with denser fibers compared to clots formed by RPs. In vitro analysis of CPs stored for 5 days also showed similar clot stiffness to that of fresh platelets. The superior fiber crosslinking in CPs could be mediated by factor XIII through binding to the activated glycoprotein IIb/IIIa on the CPs surfaces. In addition, clot histology showed that CPs had higher porosity and a more well-defined structure in their clots, similar to fresh platelets. On the other hand, RPs formed clots with altered structure and decreased porosity.
Furthermore, CPs maintained mitochondrial respiration capabilities, whereas RPs had decreased mitochondrial respiration after 7 days of storage. Finally, cold storage has also been discovered to induce significant changes in the ADP receptors of platelets. In particular, P2Y1 and P2X1 receptors decreased in CPs compared to RPs. The changes in the ADP receptors of CPs could provide an explanation as to why CPs are less responsive to inhibitory signals, such as nitric oxide and prostaglandin E1, compared to RPs. Recently, “omics” technology, such as proteomics and metabolomics, has become more accessible. This is facilitated by the decreased cost of performing these assays and the increased recognition that a large repertoire of information about platelet storage biology can be generated.
Changes in the platelet proteomes during cold storage were also examined. Different storage temperatures of platelets led to differentially expressed proteins important in platelet degranulation, blood coagulation vesicle transport, protein activation cascade, and response to stress. Storage temperature also had a significant impact on the microRNA (miRNA) expression profile of platelets. The significance of this miRNA correlation with platelet quality is still being investigated. In conclusion, cold-stored platelets are becoming an interesting research topic for many. More clinical trials are being conducted to prove the efficacy compared to room-temperature-stored platelets.
References
1.The Missing Pieces to the Cold-Stored Platelet Puzzle Hanqi Zhao1,2,3 and Dana V. Devine1,2,3,* Int J Mol Sci. 2022 Feb; 23(3): 1100.Published online 2022 Jan 20.
doi: 10.3390/ijms230311002. Transfusion News journal , Cold-Storage for Platelets December 6, 2017