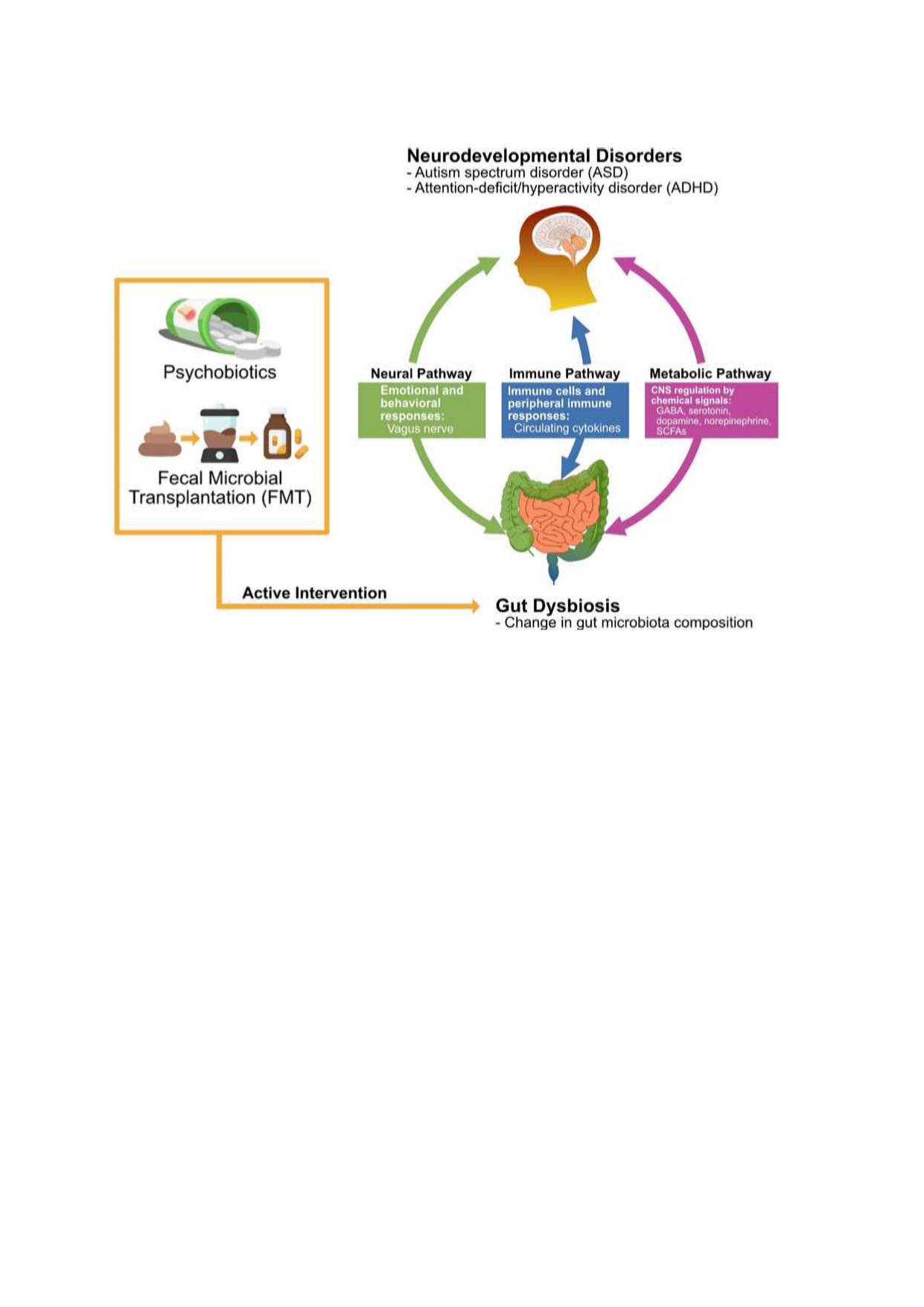
Figure 1. Gastric Brain Axis and ADHD Relationship


Innovations & Developments in Paediatric Nutritional Psychiatry: An Inspirational PhD Journey Exploring Novel Therapeutic Avenues for ADHD (PhD Candidate in AMDI/ Nutritional Psychiatry)
1. Introduction: A Convergence of Passion and Purpose
In the journey of PhD research in
pediatric nutritional psychiatry my dedication forged toward understanding how
nutritional approaches alongside innovative bioactive solutions can enhance
treatment results among children who have ADHD. At the beginning of my doctoral
educational journey, I performed a thorough systematic review that assessed
various bioactive ingredients from citicoline to trace micronutrients like
magnesium and zinc as potential treatments for ADHD symptoms. I developed the
initial research initiative which motivated me to carry out extensive work in
two important fields with distinct transformative capacities.
This initial phase illuminated several promising diet-based
interventions but also underscored that children’s responses vary considerably
based on biological, developmental, and lifestyle factors. Inspired by these
findings, I extended my research in two major directions:
a. Broadening Nutritional Interventions: Incorporating diverse dietary strategies—from the Mediterranean
diet to probiotics and synbiotics—to harness their impact on the gut-brain
axis.(Pontifex et al., 2024; Ashique et al., 2024)
b. Personalization Through Biomarkers: Investigating how genetic, metabolic, and microbial markers can guide targeted, individualized treatment plans for children with ADHD.(Haavik, 2022; Predescu et al., 2024)
2. From Systematic Review to Dual-Lens Approach
Doctors should examine dietary
approaches that utilize beneficial microorganisms specifically targeted at
treating gut-brain-axis conditions. Additionally, micronutrients used in the
regimen of ADHD have to be validated and backed up by evidence. On the other
hand, the investigation of biomarker-based treatments will create
individualized therapy methods that use a combination of biochemical values and
genetic data for developing optimal therapy designs. I present in this section
the inspirations derived from my systematic review and describe how both main
concepts advance as transformative advancements in child ADHD therapeutic
methods. FiFigure 1, below clarify the association between GBA and ADHD.
3. Inspirational Stories & Experiences:
3.1 Inspirational
Stories and Experiences
Throughout my systematic review,
many parents shared their accounts about seeking non-drug alternatives for
their children's cognitive and emotional development. Parental determination
and potential nutritional treatments motivated me to explore two main concepts
further. Researchers needed to look into the role of gut dysbiosis as a risk
factor for ADHD symptoms and the therapeutic benefits of pre-and probiotics or
synbiotics. This is why parents and doctors became interested in the Gut-Brain
Axis (GBA). A combination of concrete observations in medical settings and
scientific research data prompted teams to create two complementary strategies
for testing nutritional treatments against biomarkers that personalize
treatment plans.
3.2 Expanding the
Nutritional Horizon
My systematic review of 19 relevant
studies showed that there were big differences between the dietary treatments.
These included broad dietary patterns like the Mediterranean diet, specific
micronutrient supplementation with vitamins D and iron, zinc and magnesium, and
functional foods that contained horse milk, as well as low-lectin diets. I
devised a study schedule to identify potential dietary modifications that could
serve as effective ADHD treatments for young patients. In figure two below, the
oxidative stress and the inflammatory pathway are the primary targets of
nutritional intervention.
4. The Spark of
Personalization
Toward the end of the systematic
review, researchers found that the significance of distinguishing each
participant became a vital narrative. Children exhibited different levels of
response to dietary supplements, where some benefitted while others
demonstrated little variation in their symptoms. The different results
demonstrated the necessity for developing therapeutic strategies based on
personal factors, including metabolic traits and genetic data and biological
indicators that forecast how treatments will perform. Scientists uncovered this
enlightenment as a starting point to transform monolithic medical practices,
thus creating a precision-medicine system that provides fresh possibilities to
kids and families neglected by mainstream medicine.
4.1 Nutritional Strategies in ADHD: Evidence from Recent
Meta-Analyses
The combination of high-quality
eating habits that incorporate more fruits, vegetables, and fish consumption
helps decrease ADHD development risks in children, according to systematised
studies and statistical synthesis. The consumption of unhealthy foods with high
sugar content and junk foods produces opposite effects by increasing the
probability of ADHD occurrence (Papanastasiou et al., 2021). Research indicates
ADHD affects children who frequently eat processed foods together with sweet
treats in their diet. Research evidence shows that children who eat these diets
face heightened ADHD risk based on their elevated odds ratios (Yan et al.,
2023; Wu et al., 2023). The ADHD symptoms of children improve after taking
eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) along with vitamin D
and magnesium supplements (Rocha et al., 2024; Raczy?ska et al., 2024).
4.2 Elimination Diets and Oligoantigenic Approaches
The researchers from Walz et al.
(2022) observed that systematic elimination of common allergens through
oligoantigenic or elimination diets produced long-lasting benefits in ADHD
symptoms especially for inattention and hyperactivity/impulsivity. Annick
Huberts-Bosch et al. (2024) revealed data which indicates that although
nutritious diets produce benefits in general they provide special advantages to
people who have food sensitivities.
4.3 Microbiome-Targeted Interventions
Probiotics, Synbiotics, and Low-Lectin Diets Kefir,
Synbiotics, and Microbiome-Friendly Foods Children who consumed kefir along
with reduced sugar content and increased plant variety showed better ADHD
symptom control and overnight sleep metrics according to Lawrence et al.
(2022). When people consume synbiotics made of beneficial bacteria and
prebiotic fibers, their attention and inhibition improve significantly as their
gut bacterial populations of Faecalibacterium and Bacteroides increase,
according to Trezzi et al. (2025).
4.4 Micronutrients and Omega-3 Fatty Acids
Iron, Zinc, Vitamin D, and Magnesium The studies conducted by Noorazar et al.
(2020) and Tohidi et al. (2021) demonstrate how zinc supplements and iron
supplements effectively treat ADHD inattentiveness symptoms and related
behaviours. Evidence shows that low vitamin D levels cause ADHD symptom
deterioration, while dietary supplementation leads to decreased emotional
difficulties and less impulsivity. Research shows that when vitamin D is
combined with magnesium treatment, it delivers enhanced behavioral effects
which help minimise anxiety and social dilemmas. Research by Naeini et al.,
2019 and Dehbokri et al., 2019 supports this claim.
4.5 Omega-3 Fatty Acids
Accumulated scientific research
demonstrates the positive effects of Omega-3 fatty acids when treating ADHD
symptoms. A randomized double-blind placebo-controlled trial by Rodríguez et
al. (2019) revealed that DHA triglyceride supplementation with high DHA content
improved ADHD symptoms measuring hyperactivity along with attention deficit and
combined ADHD manifestations after six months of treatment. The research by
Martin et al. (2022) showed that omega-3 supplements effectively decreased
impulsive behavior in ADHD children.
4.6 Biomarker-Based Personalization:
4.6.1 Rationale for Precision Nutrition in ADHD
Professional nutritional guidance takes into
account genetic information to match foods to patients' requirements through
the understanding that various DNA sequences strongly impact how people process
nutrients and what substances affect them best. Vitamin and lipid metabolism in
the body is controlled by single-nucleotide polymorphisms (SNPs) (Park, 2025)
(Singar et al., 2024) such that they show how ADHD symptoms develop and how
well treatments work. The vital component of precision nutrition called
metabolomics allows scientists to detect natural individual changes from
dietary interventions, which enables them to create the most suitable diet
based on particular nutritional imbalances or deficiencies ( Metabolomics
application for the design of an optimal diet", 2022).
4.6.2 Potential Biomarkers and Implementation
Analyses of gut microbial profiles
should identify dysbiosis and low alpha diversity because this reveals suitable
choices between probiotics and synbiotics as treatments (Jung et al., 2022).
Scientific evidence demonstrates that individual mineral levels starting from
iron and lithium can predict how ADHD patients will respond to dietary
combinations. The success of dietary interventions depends on assessing the
initial iron levels in patients because patients with higher levels show
improved treatment results according to Robinette et al. (2024). The assessment
of vitamin D and copper levels in serum during the pre-treatment period does
not reliably predict treatment outcomes; nevertheless, clinical practitioners
can use this data to develop customised treatment plans. Several ADHD symptoms
show increased improvements when patients have lower blood levels of folate and
B12, according to Rucklidge et al. (2019).
5. Conclusion
Especially in paediatric populations, nutritional
psychiatry is a developing field. Rooted on the systematic review of bioactive
elements for ADHD, my PhD route has led me to investigate two transforming
research directions:
(1) gut-brain therapies using probiotics, prebiotics, and Synbiotics;
(2) tailored treatments driven by biomarkers;
(3) whole-food dietary patterns like the Mediterranean diet
alongside specific micronutrients.
These routes represent not only scientific curiosity but also a
great empathy for the families and kids confronting ADHD.
Targeting the biological underpinnings of ADHD by diet and
microbial balance and using genetic and metabolic insights for treatment
customising will help us to approach holistic, child-oriented interventions
that can either directly integrate with, or provide an alternative to, conventional
pharmacotherapy.
The motivation for this two-pronged approach ultimately comes from
seeing the resiliency and optimism of families looking for other paths—as well
as from the creativity in questioning accepted ideas to create a more complex,
statistically based, and personally relevant model of ADHD treatment.
#SDG3=Good Health and Wellbeing#
References
List:
Ashique, S., Mohanto, S., Ahmed, M.G., Mishra, N., Garg, A.,
Chellappan, D.K., Omara, T., et al. (2024), “Gut-Brain Axis: A
Cutting-Edge Approach to Target Neurological Disorders and Potential Synbiotic
Application”, Heliyon, Elsevier BV, p. e34092, doi:
10.1016/j.heliyon.2024.e34092.
Dehbokri N, Noorazar G, Ghaffari A, Mehdizadeh G, Sarbakhsh
P,Ghaffary S, Effect of vitamin D treatment in children with attention-deficit
hyperactivity disorder. World J Pediatr. 2019;15(1):78-84. https://doi.org/10.1007/s12519-018-0209-8.
Haavik, J. (2022), “Genome Guided Personalized Drug Therapy in
Attention Deficit Hyperactivity Disorder”, Frontiers in Psychiatry,
Vol. 13, doi: 10.3389/fpsyt.2022.925442.
Huberts-Bosch A, Bierens M, Ly V, van der Velde J, de Boer H, van
Beek G, et al., Short-term effects of an elimination diet and healthy diet in
children with attention-deficit/hyperactivity disorder: a randomized-controlled
trial. European Child & Adolescent Psychiatry. 2024;33(5):1503-1516.
Jung TH, Hwang HJ,Han KS, Correlation of attention deficit
hyperactivity disorder with gut microbiota according to the dietary intake of
Korean elementary school students. PLoS One. 2022;17(9):e0275520. https://doi.org/10.1371/journal.pone.0275520.
Kwak, M.-J. et al. (2023) 'Psychobiotics and fecal
microbial transplantation for autism and attention-deficit/hyperactivity
disorder: microbiome modulation and therapeutic mechanisms,' Frontiers
in Cellular and Infection Microbiology, 13. https://doi.org/10.3389/fcimb.2023.1238005.
Lawrence K, Myrissa K, Toribio-Mateas M, Minini L,Gregory AM,
Trialling a microbiome-targeted dietary intervention in children with ADHD-the
rationale and a non-randomised feasibility study. Pilot Feasibility Stud.
2022;8(1):108. https://doi.org/10.1186/s40814-022-01058-4.
Lin, S., Wu, D., Chen, S., Yan, W., Dou, L.-H. and Li, X. (2023),
“Physical growth and dietary characteristics of children with attention deficit
hyperactivity disorder: a cross-sectional study”, Vol. 25 No. 7, pp. 711–717,
doi: 10.7499/j.issn.1008-8830.2301052.
Naeini AA, Fasihi F, Najafi M, Ghazvini MR,Hasanzadeh A, The
effects of vitamin D supplementation on ADHD (Attention Deficit Hyperactivity
Disorder) in 6–13 year-old students: A randomized, double-blind,
placebo-controlled study. European Journal of Integrative Medicine.
2019;25:28-33.
Noorazar SG, Malek A, Aghaei SM, Yasamineh N,Kalejahi P, The
efficacy of zinc augmentation in children with attention deficit hyperactivity
disorder under treatment with methylphenidate: A randomized controlled trial. Asian
J Psychiatr. 2020;48:101868. https://doi.org/10.1016/j.ajp.2019.101868.
Papanastasiou, G., Drigas, A. and Papanastasiou, P. (2021), “The
association of diet quality and lifestyle factors in children and adults with
ADHD: a systematic review and meta-analysis”, Scientific Electronic
Archives, Scientific Electronic Archives, Vol. 14 No. 9, doi:
10.36560/14920211441.
Park, S. (2025), “Editorial: Precision nutrition and nutrients:
making the promise a reality”, Frontiers in Nutrition, Frontiers
Media, Vol. 12, doi: 10.3389/fnut.2025.1553149.
Pontifex, M.G., Connell, E., Le Gall, G., Láng, L., Pourtau, L.,
Gaudout, D., Angeloni, C., et al. (2024), “A novel
Mediterranean diet-inspired supplement ameliorates cognitive, microbial, and
metabolic deficits in a mouse model of low-grade inflammation”, Gut
Microbes, Landes Bioscience, Vol. 16 No. 1, doi:
10.1080/19490976.2024.2363011.
Predescu, E., Vaidean, T., Rapciuc, A.-M. and Sipos, R. (2024),
“Metabolomic Markers in Attention-Deficit/Hyperactivity Disorder (ADHD) among
Children and Adolescents—A Systematic Review”, International Journal of
Molecular Sciences, doi: 10.3390/ijms25084385.
Raczy?ska, A., Popczy?ska, J., Pacocha, N., Krzemie?, O., Kosiec,
K., Jedrychowski, J.R., Karpowicz, N., et al. (2024), “Omega-3
fatty acid supplementation for attention deficit hyperactivity disorder in
children and adolescent: literature review”, International Journal of
Innovative Technologies in Social Science, RS Global Sp. z O.O., No. 2(42),
doi: 10.31435/rsglobal_ijitss/30062024/8153.
Robinette, L.M. (2024), “Evaluating Mineral Biomarkers as Mediators
and Moderators of Behavioural Improvements in a Randomized Controlled Trial of
Multinutrients for Children with ADHD”, British Journal of Nutrition,
Cambridge University Press, pp. 1–38, doi: 10.1017/s0007114524001132.
Rocha, M. da S.P., de Aquino, L.L., Gonçalves, G. de O., Barbosa,
S.M.V., de Freitas, J.L., Lopes, V.B., Dias, W.C., et al. (2024),
“Major clinical implications of adequate nutrition in children and adolescents
with attention-deficit/hyperactivity disorder: a concise systematic
review”, International Journal of Nutrology, doi:
10.54448/ijn24205.
Rodríguez C, García T, Areces D, Fernández E, García-Noriega
M,Domingo JC, Supplementation with high-content docosahexaenoic acid triglyceride
in attention-deficit hyperactivity disorder: a randomized double-blind
placebo-controlled trial. Neuropsychiatr Dis Treat. 2019;15:1193-1209. https://doi.org/10.2147/ndt.s206020.
Rucklidge, J.J., Eggleston, M.J.F., Darling, K.A., Stevens, A.J.,
Kennedy, M.A. and Frampton, C. (2019), “Can we predict treatment response in
children with ADHD to a vitamin-mineral supplement? An investigation into
pre-treatment nutrient serum levels, MTHFR status, clinical correlates and
demographic variables.”, Progress in Neuro-Psychopharmacology &
Biological Psychiatry, Prog Neuropsychopharmacol Biol Psychiatry, Vol. 89,
pp. 181–192, doi: 10.1016/J.PNPBP.2018.09.007.
San Mauro Martin I, Sanz Rojo S, González Cosano L, Conty de la
Campa R, Garicano Vilar E,Blumenfeld Olivares JA, Impulsiveness in children
with attention-deficit/hyperactivity disorder after an 8-week intervention with
the Mediterranean diet and/or omega-3 fatty acids: a randomised clinical trial.
Neurologia (Engl Ed). 2022;37(7):513-523. https://doi.org/10.1016/j.nrleng.2019.09.009.
Schleupner, H.V. and Carmichael, M.J. (2022)
'Attention-Deficit/Hyperactivity Disorder and the Gut Microbiota–Gut–Brain
Axis: Closing Research Gaps through Female Inclusion in Study Design,' Women,
2(3), pp. 231–253. https://doi.org/10.3390/women2030023.
Singar, S., Nagpal, R., Arjmandi, B. H., & Akhavan, N. (2024).
Personalized Nutrition: Tailoring Dietary Recommendations through Genetic
Insights. Nutrients, 16(16), 2673. https://doi.org/10.3390/nu16162673
Tohidi S, Bidabadi E, Khosousi MJ, Amoukhteh M, Kousha M, Mashouf
P, et al., Effects of Iron Supplementation on Attention Deficit Hyperactivity
Disorder in Children Treated with Methylphenidate. Clin Psychopharmacol
Neurosci. 2021;19(4):712-720. https://doi.org/10.9758/cpn.2021.19.4.712.
Trezzi S, Scaccabarozzi G, Nossa R, Piazza C, Bianchi AR, Rosi E,
et al., Behavioural, cognitive, and neurophysiological effects of a synbiotic
supplementation enriched with pigmented corn extract or cornstarch in
drug-naïve children with attention-deficit hyperactivity disorder: A
randomised, double-blind, comparison-controlled clinical trial. Clin Nutr
ESPEN. 2025;65:408-417. https://doi.org/10.1016/j.clnesp.2024.12.016.
Walz G, Blazynski N, Frey L, Schneider-Momm K, Clement HW, Rauh R,
et al., Long-Term Effects of an Oligoantigenic Diet in Children with
Attention-Deficit/Hyperactivity Disorder (ADHD) on Core Symptomatology. Nutrients.
2022;14(23):1-10. https://doi.org/10.3390/nu14235111.
Wu, Y., Lin, S., Wu, D., Shi, Y., Dou, L. and Li, X. (2023),
“Processed Food–Sweets Patterns and Related Behaviors with Attention Deficit
Hyperactivity Disorder among Children: A Case–Control Study”, Nutrients,
Vol. 15 No. 5, p. 1254, doi: 10.3390/nu15051254.